Sunday, May 9, 2010
The control of CO2 emission is becoming one of the most challenging environmental issues facing many countries today. Recent years has seen an increase in the reported use of process simulators to assess feasibility and design and troubleshoot of CO2 capture using amine solution. A simulation-optimization framework comprising of HYSYS simulator is presented and a jumping gene based multi-objective simulated annealing technique to evaluate the efficacy of CO2 removal using DEA solution. The novelty of this approach is that, both simulation and optimization of the process are performed simultaneously in an automated fashion to fully explore the trade-off surface of CO2 capture efficiency and operating cost.
The framework has been developed by integrating HYSYS simulator with a jumping gene based multi-objective simulated annealing technique and applied to CO2 capture process from a gas power plant. It has been shown to be capable of generating Pareto optimal solution involving CO2 capture efficiency and operating cost. Such Pareto set will form the basis for comparison with other amine technology. Future work include using other amine solutions as well as mixture of amines. Application to coal or fuel based power plant will also be part of future investigation.
DownloadRelated Topics
- Performance of Gas Sweetening with Mixture of DEA & MDEA
- HYSYS Simulation of MEA Based CO2 Removal
- Amines Type & Points Assist in Selection
- Several Concerns in High CO2 Field Development
- Correct model and thermo package in Amine system simulation using HYSYS
- Safety Moment with H2S
- What are the concerns related to H2S ?
- CO2 Related
Labels: Acid Gas Removal, CO2, Gas Sweethening
Tuesday, May 4, 2010
Methyldiethanolamine (MDEA), and Diethanolamine (DEA) have been used for CO2 & H2S removal in Gas sweetening unit. DEA is non-selective to CO2 and H2S. However, irreversible reactions of DEA with CO forming corrosive degradation products & increase corrosiveness. MDEA is highly selective to H2S with low corrosiveness. What is performance of mixtures of DEA and MDEA solutions in CO2 and H2S removal ?
In the present paper, the use of amine mixtures employing methyldiethanolamine (MDEA), and diethanolamine (DEA) have been investigated for a variety of cases using a ASPEN HYSYS process simulation. The simulation results show that,
- 40%MDEA with 10% DEA, 30% MDEA with 10 % DEA and 40% MDEA with 5% DEA were the best result in the absorption processes of CO2 from the natural gas.
- 30%MDEA with 10% DEA, 40%MDEA with 5 % DEA and 40%MDEA with 10 % DEA were the best result in the absorption processes of H2S from the natural gas comparing with other mixing amines.
- 20% MDEA with 10% DEA, 30% MDEA with 10 % DEA and the 40% MDEA with 10 % DEA have the lowers amount of H2S in the sweet gas comparing with different percent of DEA without mixing.
- The temperature of rich amine decrease with increase the circulation rate in all different percent of mixing amines and different percent of MDEA without mixing.
- The reboiler temperature was constant in the 20% MDEA with 10 % DEA and in the 30%MDEA, 40%MDEA and in the 50%MDEA, otherwise the reboiler temperature increase with increase the circulation rate in all different percent of mixing amines.
Related Topics
- HYSYS Simulation of MEA Based CO2 Removal
- Amines Type & Points Assist in Selection
- Several Concerns in High CO2 Field Development
- Correct model and thermo package in Amine system simulation using HYSYS
- Safety Moment with H2S
- What are the concerns related to H2S ?
- CO2 Related
Labels: Acid Gas Removal, CO2, Gas Sweethening
Monday, May 3, 2010
MEA (monoethanol amine) based CO2 removal process have been simulated with the ASPEN HYSYS process simulation tool with the Peng Robinson (PR) and Amines Property Package models which are available in Aspen HYSYS. The CO2 removal in % and the energy consumption in the CO2 removal plant are calculated as a function of amine circulation rate, absorption column height, absorption temperature and steam temperature.
Recommended :
Subscribes to FREE Hydrocarbon Processing
 Selection of type of solvent is complicated and subject to many parameters such feed gas composition and condition, gas impurities specification, life cycle cost, space, salt deposition, byproduct, losses, hydrocarbon absorption, etc. Read more in "Amines Type & Points Assist in Selection".
Selection of type of solvent is complicated and subject to many parameters such feed gas composition and condition, gas impurities specification, life cycle cost, space, salt deposition, byproduct, losses, hydrocarbon absorption, etc. Read more in "Amines Type & Points Assist in Selection".
Recommended :
Subscribes to FREE Hydrocarbon Processing
An absorption and desorption process for CO2 removal with an aqueous MEA solution has been simulated. The exhaust gas from the power plant model is used as the feed to this model. The absorption column is specified with 10 stages each with a Murphy efficiency of 0.25. An estimated HETP (Height Equivalent to a Theoretical plate) of 4 meter, is about equivalent to 0.25 efficiency for each meter of packing. Traditional concentrations, temperatures and pressures are used in the base case simulation. The thermodynamics for this mixture is described by an Amines Property Package available in Aspen HYSYS. The Kent Eisenberg model is selected in the Amines Property Package. The Aspen HYSYS CO2 removal model is presented below.
With CO2 removal of 85 %, heat consumption is calculated to 3.7 MJ/kg CO2 removed, close to a literature value of 4.0 MJ/kg CO2.
Related Topics
- Amines Type & Points Assist in Selection
- Several Concerns in High CO2 Field Development
- Correct model and thermo package in Amine system simulation using HYSYS
- Safety Moment with H2S
- What are the concerns related to H2S ?
- CO2 Related
Labels: Acid Gas Removal, CO2, Gas Sweethening
Saturday, December 20, 2008
Display problem ? Click HERE
Recommended :
Subscribes to FREE Hydrocarbon Processing
 Present of Hydrogen Sulfide (H2S) and Carbon Dioxide (CO2) in wet natural gas will cause severe metal stress cracking and corrosion possibly leads to severe leakage. Besides corrosion and stress cracking issue, these contaminant may need to be removed to meet gas specification. The removal of H2S from natural gas is common referred as Gas Sweetening.
Present of Hydrogen Sulfide (H2S) and Carbon Dioxide (CO2) in wet natural gas will cause severe metal stress cracking and corrosion possibly leads to severe leakage. Besides corrosion and stress cracking issue, these contaminant may need to be removed to meet gas specification. The removal of H2S from natural gas is common referred as Gas Sweetening.
One of the process of removal of H2S and CO2 is by solvent absorption where CO2 and H2S is react with solvent. There are many type of solvents available the market :
Subscribes to FREE Hydrocarbon Processing
One of the process of removal of H2S and CO2 is by solvent absorption where CO2 and H2S is react with solvent. There are many type of solvents available the market :
- Monoethanolamine (MEA)
- Diethanolamine (DEA)
- Diisopropanolamine (DIPA)
- Diglycolamine (DGA)
- Triethanolamine (TEA)
- Methyldiethanolamine (MDEA).
- Special solvent i.e. Activated / Accelerated MDEA
- Sterically Hindered Amines
- Physical solvent
Amine Types & Selection Guide Points
Selection of type of solvent is complicated and subject to many parameters such feed gas composition and condition, gas impurities specification, life cycle cost, space, salt deposition, byproduct, lossess, hydrocarbon absorption, etc. Following are some characteristics and guide points may be referred.
Selection of type of solvent is complicated and subject to many parameters such feed gas composition and condition, gas impurities specification, life cycle cost, space, salt deposition, byproduct, lossess, hydrocarbon absorption, etc. Following are some characteristics and guide points may be referred.
MEA
- react most rapid with acid gases
- faster reaction with H2S compare to CO2
- remove Co2 & H2S
- non-selective between CO2 & H2S
- low acid gas partial pressures
- low absorber pressure
- stringent acid gas specification : H2S lower than 4.0 ppmv
- stringent acid gas specification : CO2 lower than 100 ppmv (low to moderate pressures)
- irreversible reaction MEA with Carbonyl Sulfide (COS) & Carbon Disulfide (CS2) lead to MEA losses & contamination
- typical pickup : 0.3-0.4 moles of acid gas/mole of MEA
- typical solution concentration 10-20 wt%
- higher concentration (more 20 wt%) increase CO2 loading in MEA. Potential high corrosion
- high reaction heat lead to high energy consumption for stripping
- Low vapor pressure ease vaporisation losses. Water wash unit to minimize losses.
DEA
- general purposes
- non-selective between CO2 & H2S
- good for moderate pressure compare to MEA
- typical pickup 0.2-0.8 of acid gas/mole of DEA
- typical solution concentration 10-20 wt%
- Special SNPA-DEA process solution concentration can be more than 30 wt%
- Special SNPA-DEA process claimed to have 0.70 to 1 .0 mole of acid gas / mole of DEA
- forms regenerable compound with COS and CS2
- Slower reaction with COS & CS2 lead to less regenerable compound with these component
- no significant amount of nonregenerable
- irreversible reactions with CO2, forming corrosive degradation products & increase corrosiveness of amine solution
DGA
- removal of H2S, CO2, COS and mercaptans
- proprietary process
- high affinity for absorption of aromatics, BTEX, olefins, and heavy hydrocarbons (potentially foaming & tail gas treatment for BTEX)
- may be used at low pressure system i.e 8.6 barg
- typical pickup 0.25-0.38 of acid gas/mole of DGA
- typical solution concentration 50-60 wt%
- low freezing point good for low climate application compare to MEA & DEA (link to typical concentration)
MDEA
- high selective to H2S at moderate to high pressure which provides added advantages i.e reduced solvent flow rates, smaller unit, etc.
- H2s & CO2 may be partially removed from MDEA by flash. Less heating required during regeneration
- typical solution concentration 30-50 wt%
- typical pickup 0.2-0.8 of acid gas/mole of MDEA
Special Solvent (Activated / Accelerated MDEA)
- Licensed solvent and process
- Required licensing fees.
- Some Lisensor mandatory licensee to purpose solvent from lisensor
- much lower circulation rate
- small unit
- less heating & cooling
- lower corrosion
- Licensor : INEOS, Huntsman, Dow Chemical, UOP, SGS, Prosenat, BASF,


IAcid and/or Sour Gas Absorption Process
Acid / Sour gas prior flow into the amine absorver, it normally will pass throught a separator in order to remove solid and liquid from the gas. In some of the unit, a wash water is circulated to increase the solid, entrained liquid from gas to avoid potential foaming in the absorber.
The acid / sour gas is run through a absorber and contacts with amine solution. Absorption follow by reaction between acid / sour gas component (CO2, H2S, COS, CS2, mercaptant) will take place. Reacted amines normally known as Rich Amine will be regenerated in Amine regeneration unit. The absorber is normally a tray column. Packing column or mix of packaing and tray are used for some services.
Following is a video clip for Principle of Amine Sweetening. It described the mechanism take place in the abosorber.
References :
i) GPSA
ii) "Gas Purifcation" by Arthur Kohl & Richard Nielsen
iii) Cambells Gas Conditioning & Processing Vol 4.
The acid / sour gas is run through a absorber and contacts with amine solution. Absorption follow by reaction between acid / sour gas component (CO2, H2S, COS, CS2, mercaptant) will take place. Reacted amines normally known as Rich Amine will be regenerated in Amine regeneration unit. The absorber is normally a tray column. Packing column or mix of packaing and tray are used for some services.
Following is a video clip for Principle of Amine Sweetening. It described the mechanism take place in the abosorber.
References :
i) GPSA
ii) "Gas Purifcation" by Arthur Kohl & Richard Nielsen
iii) Cambells Gas Conditioning & Processing Vol 4.
Labels: Acid Gas Removal, gas processing, Gas Sweethening
Monday, October 8, 2007
Above image shows a typical flow sheets for oil and gas exploration, Liquefied natural gas (LNG), Liquefied Petroleum Gas (LPG) and condensate production.
General units involved are Oil & gas exploration (sub-sea, top site, onshore), bulk separation, gas treatment prior to Natural Gas Liquid (NGL) extraction, Liquefied Petroleum Gas (LPG) fractionation, condensate stabilization, Carbon Dioxide (CO2) gas compression & reinjection, Mono-Ethylene glycol (MEG) regeneration, etc.
The following flash shows all above in a very simple manner. It is very useful to all engineers who just get involved in Oil & gas business. Click HERE for detail text.
Related Topics :
General units involved are Oil & gas exploration (sub-sea, top site, onshore), bulk separation, gas treatment prior to Natural Gas Liquid (NGL) extraction, Liquefied Petroleum Gas (LPG) fractionation, condensate stabilization, Carbon Dioxide (CO2) gas compression & reinjection, Mono-Ethylene glycol (MEG) regeneration, etc.
The following flash shows all above in a very simple manner. It is very useful to all engineers who just get involved in Oil & gas business. Click HERE for detail text.
Related Topics :
Labels: Acid Gas Removal, Fractionation, gas processing, Gas Sweethening, Refinery
Tuesday, September 4, 2007
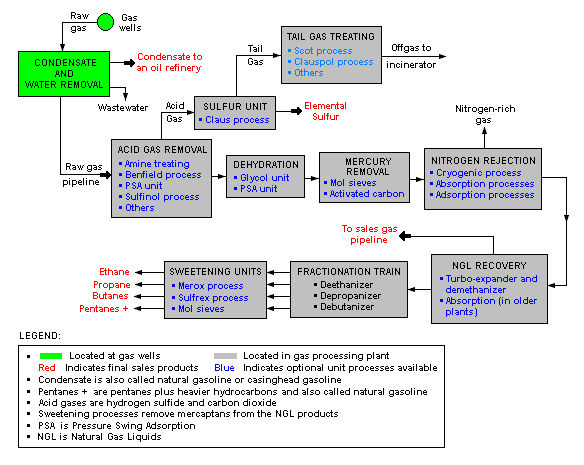
Milt Beychok from www.air-dispersion.com has shared a schematic flow diagram of a typical GAS PROCESSING that depicts the various unit processes and the flow of intermediate product streams that occurs between the inlet Gas with associate condensate as feedstock and the final end products. This image has given a brief idea how gas/condensate goes through separation and purification. Products included Condensate, C2, C3, C4, Light gas (high C1), and side product elementary Sulfur.
Click image to view original
Labels: Acid Gas Removal, gas processing, Gas Sweethening
Monday, July 9, 2007

Looking at this particular logo, i am sure many of you know what it mean...
BR&E - Bryan Research & Engineering Inc, has been in the oil and gas industry for years. My first contact with BR&E was 10 years ago.
Bryan Research & Engineering incepted in 1974, renowed process simulation software, ProMax having its ability to predict the performance of gas processing, refining and petrochemical processes.

ProMax capabilities includes Amine Sweetening, Glycol Dehydration, Equipment Rating/Sizing, Crude Oil Refining, LPG Recovery and Caustic Treating.
FREE articles (AMINE related) available BR&E site :
Addition of Static Mixers Increases Treating Capacity in Central Texas Gas Plant
Alternative Flow Schemes to Reduce Capital and Operating Costs of Amine Sweetening Units
Analysis of Amine Solutions by Gas Chromatography
Analysis of Various Flow Schemes for Sweetening with Amines
Converting to DEA/MDEA Mix Ups Sweetening Capacity
Decreasing Contactor Temperature Could Increase Performance
Design & Operation of a Selective Sweetening Plant Using MDEA
Design Alternatives for Sweetening LPG's and Liquid Hydrocarbons with Amines
Dome's North Caroline Plant Successful Conversion to MDEA
Improved Absorber-Stripper Technology for Gas Sweetening to Ultra-Low H2S Concentrations
Influence of Ammonia on Gas Sweetening Units Using Amine Solutions
Optimization of Amine Sweetening Units
Optimization of New and Existing Amine Gas Sweetening Plants Using Computer Simulation
Selecting Amines for Sweetening Units
Selective Absorption Using Amines
Solubility of Hydrocarbons in Physical Solvents
Sweetening LPG's with Amines
The Use of MDEA and Mixtures of Amines for Bulk CO2 Removal
Treat LPGs with Amines
Unique Acid Gas Enrichment Application
Using Mixed Amine Solutions for Gas Sweetening
The Impact Of Acid gas Loading On The Heat Of Absorption And VOC and BTEX Solubility in Amines Sweetening units
If this post ibenefits to you...buy me some sweets
Labels: Acid Gas Removal, Amine, Design, Gas Sweethening, Process simulation, ProMax
Sunday, July 8, 2007

Somedays ago...somebody raise a very simple question. He is doing revamping of existing amine absorber column. This column contains trayed and packed bed section. He is using HYSYS process simulator. How shall he simulate it in HYSYS ?
Yeah...probably a very simple question to those who has experiences with HYSYS. I know i can simply response to him saying that please refer to HYSYS manual...but still i take a little of my time to elaborate.
Amines absorber column may contains trays and packing for some design reasons...Can HYSYS simulate combined tray and packed bed column ?
Yes...you may do so with following steps...
- Go to Tools/Utilities
- Select "Tray sizing" option
- Choose the tower in your flowsheet that you want to size, add (define) two column sections - one for trays and other for packing.
- In the Design tab, from drop-down list find the packing type that matches your tower internals. There are additional packing data shall be enterred.
I purpose pack this information here for ease of everybody...
Further reading
If you benefits from this post...buy me some sweets...
Labels: Acid Gas Removal, Gas Sweethening, HYSYS, Process simulation
Saturday, July 7, 2007

Amine is a solvent widely used in removal of H2S and CO2 in natural gas. They are generally called gas sweetening and acid gas removal. Generally H2S and CO2 absorb and react with amine in the absorption tower will then be regenerated in the regeneration tower. Regenerated amine will then return back to absorption tower. H2S and CO2 flashed from regeneration tower will be send to thermal oxidizer (TO) and it will then be destroyed in the TO. In case the H2S is significant, the H2S will normally sent to Sulfur Recovery Unit (SRU) to recover Sulfur as byproduct.
There are number of software such as ASPEN HYSYS, SULSIM, PROMAX, AMSIM, etc available in the market which have been widely used by process engineer to simulate the entire amine loop. In HYSYS, there are Li-Mather model and Kent-Eisenberg model available for amine system. Which model shall be used ? There are ideal and non-ideal thermo package, which thermo should be used ?
Li-Mather is a fundamental and rigorous model whilst Kent-Eisenberg method is more empirical. With sufficient study and field data backup with Li Mather model and limitation of Kent-Eisenberg method (limited working envelop), it is advisable to use Li-Mather method for design purpose. Designer can always advisable to counter check with Kent-Eisenberg method.
The non-ideal themo package is taking into accounts of kinetic effects. Hence, non-ideal thermo package is generally adopted in the event feed contains significant amount of CO2 (slow reaction). As for feed dominant with H2S, then they should be minimal impact to the results as the reaction is fast.
Labels: Acid Gas Removal, Gas Sweethening, HYSYS, Sulfur Recovery