Sunday, September 9, 2007
In one of the field development project, the feed composition contain trace amount of hydrogen (H2). What extra pre-cautions that we needs to take care as compare to other hydrocarbon gases? One of the problems that shall always be taken care is Hydrogen embrittlement and material selection.
What is H2 embrittlement ?
H2 embrittlement is the embrittlement of metal or alloy involves hydrogen ingression in to metal or alloys matrix and significantly decrease it’s ductility (ability to deform), cause metal or alloys crack and catastrophic failure at stresses below normal yield stress level of the attacked material.
How H2 cause metal or alloys lose it ductility ?
Whenever metal or alloys expose to fluid contains hydrogen, hydrogen will dissolved in solid metal to give up it electrons to metal and form hydrogen nucleus which is very small. Small nucleus hydrogen will stay in the metal or alloys matrix, it probably will meet with another nucleus hydrogen and combine and form molecule Hydrogen. Molecule Hydrogen will expand and cause metal or alloys cracking in micro scale. This partially reduce it tensile strength. Expansion of molecule Hydrogen in metal or alloys matrix will displaced it metal further and decrease its ductility.
How H2 is introduce into metal or alloy matrix ?
Hydrogen can be introduced into metal or alloy :
- manufacturing & making of metal or alloy
- welding
- contact with fluid contains hydrogen
- electroplating
- cathodic protection
- rusting (corrosion)
- phosphating
- pickling
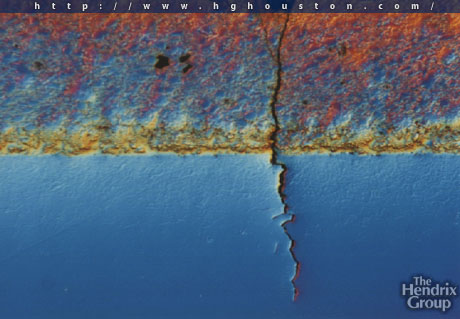
Metal cracking cause by H2 embrittlement
Following are some images shows cracking caused by h2 embrittlement.
How to minimize or avoid H2 embrittlement ?
There are number of ways to minimize or avoid H2 embrittlement :
a) minimize amount of residual H2 in metal or alloy during fabrication
- Cleaning, preheating, keep dry during welding
- avoid rapid heating and cooling
- Post Weld Heat Treatment of metal or alloy
b) minimize amount of H2 pick up by metal or alloy during operation
- avoid using chemical or additive generating H2
c) Use high resistance to H2 embrittlement material e.g. aluminium, plastic, etc
d) Use low strength metal or alloy
e) Use plating or coating process with low or no hydrogen generation
Further reading
- Different equation for Pitting Resistance Equivalent Number (PREN)
- Chloride stress corrosion cracking and use of correct MOC for seawater
- Pitting corrosion - Mechanism & Prevention
- Hydrogen Embrittlement : A guide for the metal finisher
- Hydrogen embrittlement : How Small Details Can Have Large Effects
Labels: Corrosion, Corrosion Resistance Material, Material


0 Comments:
Post a Comment
Let us know your opinion !!! You can use some HTML tags, such as <b>, <i>, <a>
Subscribe to Post Comments [Atom]
Home:
<< Home