Wednesday, September 2, 2009
Display problem ? Click HERE
Recommended :
- Subscribe FREE - Chemical Processing
- Tips on Succession in FREE Subscription

Earlier post "Estimate Mixture Flammability & Explosivity At Reference P & T" discussed about the Lower flammable limit (LFL) or Lower Explosive Limit (LEL) and Upper flammable limit (UFL) or Upper Explosive Limit (UEL) for single component fluid and mixture at reference pressure (Pref) and temperature (Tref). This post will discuss the way to correlate the LFL/LEL and UFL/UEL at Pref and Tref and operating pressure (P) and temperature (T) .
Temperature & Pressure Corrected LFL/LEL & UFL/UEL
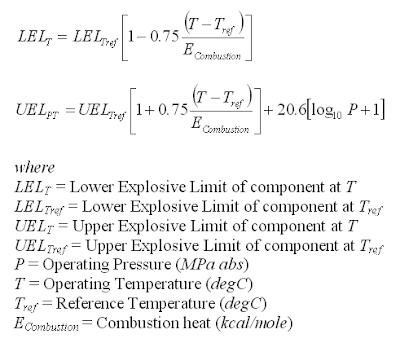
Below are two equations may be used to correlate the LFL/LEL and UFL/UEL at Pref and Tref and operating pressure (P) and temperature (T).
One shall take note that these equations are used for single component. For a mixtures, the LFL/LEL and UFL/UEL at operating pressure (P) and temperature (T) will be calculated using following equation (as discussed in "Estimate Mixture Flammability & Explosivity At Reference P & T":
Calculation Steps
2 steps in determining mixtures LFL/LEL and UFL/UEL at operating pressure (P) and temperature (T) .
(i) Estimate LFL/LEL and UFL/UEL at operating pressure (P) and temperature (T) for every component in a mixture
(ii) Estimate mixture LFL/LEL and UFL/UEL
Example
A mixture contains of Methane, Ethane and Propane with volume% of 20%, 20% and 60%. Estimate UEL at (i) 20 degC & 101.325 Pa, (ii) 70 degC & 3 MPa.
Data
Methane (C1)
UELC1,20C,1ATM = 15.0%, EC1,combustion = 212.79 kcal/mole
Ethane (C2)
UELC2,20C,1ATM = 12.4%, EC2,combustion = 372.81 kcal/mole
Propane (C3)
UELC3,20C,1ATM = 10.1%, EC3,combustion = 526.74 kcal/mole
Output
(i) UELMix at 20 degC & 101.325 Pa
(ii) UELMix at 70 degC & 3 MPa
UELC1,7oC,3MPa
= 15 x [1+0.75(70-20)/212.79]
+ 20.6 x [Log10(3)+1]
= 48.07 vol%
UELC2,7oC,3MPa
= 12.4 x [1+0.75(70-20)/372.81]
+ 20.6 x [Log10(3)+1]
= 44.08 vol%
UELC3,7oC, 3MPa
= 10.1 x [1+0.75(70-20)/526.74]
+ 20.6 x [Log10(3)+1]
= 41.25 vol%
UELMix,70C,3MPa = 1 / [ 0.2/48.07 + 0.2 /44.08 + 0.6 / 41.25 ]
Related Topic
- Subscribe FREE - Chemical Processing
- Tips on Succession in FREE Subscription

Earlier post "Estimate Mixture Flammability & Explosivity At Reference P & T" discussed about the Lower flammable limit (LFL) or Lower Explosive Limit (LEL) and Upper flammable limit (UFL) or Upper Explosive Limit (UEL) for single component fluid and mixture at reference pressure (Pref) and temperature (Tref). This post will discuss the way to correlate the LFL/LEL and UFL/UEL at Pref and Tref and operating pressure (P) and temperature (T) .
Temperature & Pressure Corrected LFL/LEL & UFL/UEL
Below are two equations may be used to correlate the LFL/LEL and UFL/UEL at Pref and Tref and operating pressure (P) and temperature (T).
One shall take note that these equations are used for single component. For a mixtures, the LFL/LEL and UFL/UEL at operating pressure (P) and temperature (T) will be calculated using following equation (as discussed in "Estimate Mixture Flammability & Explosivity At Reference P & T":
Calculation Steps
2 steps in determining mixtures LFL/LEL and UFL/UEL at operating pressure (P) and temperature (T) .
(i) Estimate LFL/LEL and UFL/UEL at operating pressure (P) and temperature (T) for every component in a mixture
(ii) Estimate mixture LFL/LEL and UFL/UEL
Example
A mixture contains of Methane, Ethane and Propane with volume% of 20%, 20% and 60%. Estimate UEL at (i) 20 degC & 101.325 Pa, (ii) 70 degC & 3 MPa.
Data
Methane (C1)
UELC1,20C,1ATM = 15.0%, EC1,combustion = 212.79 kcal/mole
Ethane (C2)
UELC2,20C,1ATM = 12.4%, EC2,combustion = 372.81 kcal/mole
Propane (C3)
UELC3,20C,1ATM = 10.1%, EC3,combustion = 526.74 kcal/mole
Output
(i) UELMix at 20 degC & 101.325 Pa
UELMix = 1 / [ 0.2/15 + 0.2 /12.4 + 0.6 / 10.1 ]
UELMix = 11.25 vol% at 20 degC & 101.325 kPaA
(ii) UELMix at 70 degC & 3 MPa
UELC1,7oC,3MPa
= 15 x [1+0.75(70-20)/212.79]
+ 20.6 x [Log10(3)+1]
= 48.07 vol%
UELC2,7oC,3MPa
= 12.4 x [1+0.75(70-20)/372.81]
+ 20.6 x [Log10(3)+1]
= 44.08 vol%
UELC3,7oC, 3MPa
= 10.1 x [1+0.75(70-20)/526.74]
+ 20.6 x [Log10(3)+1]
= 41.25 vol%
UELMix,70C,3MPa = 1 / [ 0.2/48.07 + 0.2 /44.08 + 0.6 / 41.25 ]
UELMix,70C,3MPa = 43.02 vol% at 70 degC & 3 MPaA
Related Topic


0 Comments:
Post a Comment
Let us know your opinion !!! You can use some HTML tags, such as <b>, <i>, <a>
Subscribe to Post Comments [Atom]
Home:
<< Home